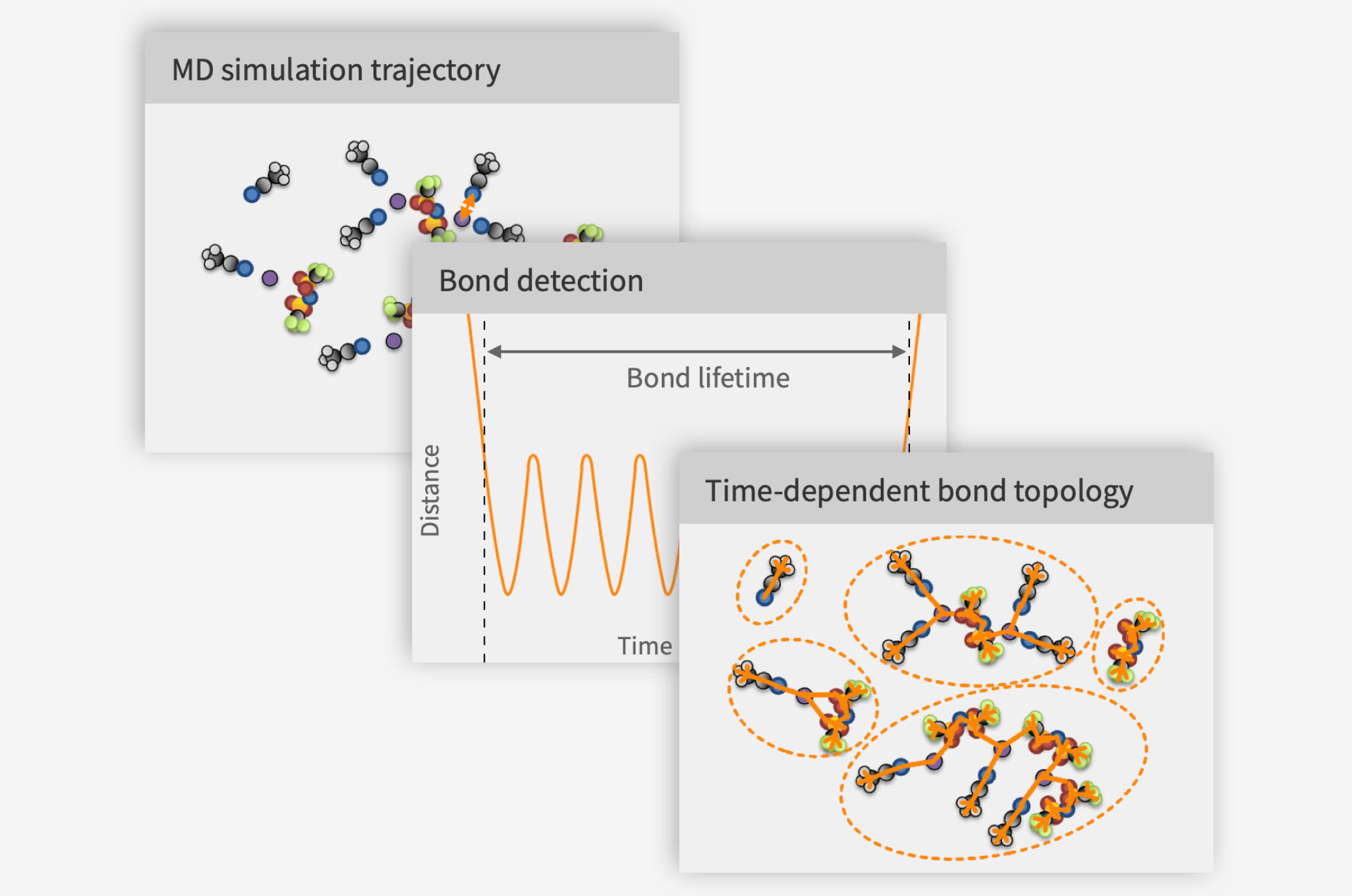
As batteries such as lithium-ion batteries are cycled, some solvent components are reduced to form the solid-electrolyte interphase (SEI). While this process is most rapid in the first few cycles, it is also an important factor in the long-term aging of the cell. As some electrolyte species (typically ethylene carbonate, EC) preferentially break down, their concentrations dwindle and the overall electrolyte composition changes, which affects the physicochemical properties of the electrolyte as well as the performance of the cell as a whole.
In most cell models, these effects are entirely neglected, as the electrolyte is assumed to be well described by a single value for each of the relevant transport properties, but in reality the electrolyte properties vary wildly across the cell and throughout operation as functions of salt concentration, temperature, and solvent composition. Here, we present how including the rheological effects of solvent breakdown alters the predictions of a pseudo-2D Doyle-Fuller-Newman model (P2D/DFN) with aging through EC consumption and SEI formation, as implemented in COMSOL Multiphysics.
The results presented here come from a study performed by Compular together with COMSOL to showcase the synergies between their Battery Design Module in COMSOL Multiphysics and our Compular Lab web application in building physically accurate and predictive cell models beyond the previous state-of-the-art. We show that capturing the effects of the change of solvent composition as the cell ages gives meaningfully different predictions both qualitatively and quantitatively for predicting and understanding the aging of the cell.
Sourcing the parameters
The electrolyte material parameters needed for the aging model are not trivial to come by.
The obvious source for electrolyte properties would be peer-reviewed journal papers, or materials databases, such as the one provided in COMSOL Multiphysics. Unfortunately, available sources rarely provide data with variations in both salt concentration and solvent composition.
The second most natural source of parameters would be to measure them yourself. If you would like to measure the electrolyte properties needed for a P2D or similar model in the lab, you will need to determine four distinct properties for each composition:
- ionic conductivity,
- salt diffusivity,
- cation transference number,
- thermodynamic factor (or equivalently activity coefficient).
While ionic conductivity is easy enough to measure by electrochemical impedance spectroscopy (EIS), the other properties are significantly more difficult to measure accurately. Not least the transference number, for which every broadly available experimental technique (e.g. Bruce-Vincent and PFG NMR) relies on assumptions that are never met in practical lithium-ion battery electrolytes, making the results less than reliable.
A third option involves fitting the electrolyte parameters along with those for electrode properties from a set of cell experiments. There are two main problems with this approach. First, the commonly available combination of experiments (cell charge/discharge protocols at varying C-rates and pulses, sometimes complemented by EIS) leaves the set of parameters under-determined. This means that you will probably be able to get a good fit to any nominal charge/discharge protocol, but you will very likely fail to capture the correct changes to the transport properties, which makes your model liable to break down and give unphysical results when you go outside the regime for which it was fitted. Secondly, you will need to re-fit the model many times over throughout the aging process which takes a lot of both work and time, and once again, you can’t expect to distinguish accurately between different aging processes and how they affect the model parameters.
A more powerful alternative
Molecular dynamics (MD) offers an alternative: predicting the electrolyte properties from first principles. MD essentially entails solving Newton’s equations for large systems of simulated atoms and molecules. This generates big amounts of data on how individual atoms move. This data can be crunched to extract material properties, such as density, viscosity and, of relevance for electrolytes, ion transport properties. The interactions between atoms are handled by classical physics, but the strengths of different interactions come from quantum chemistry calculations, which can be used to predict atomic and molecular properties from our knowledge of fundamental physics. This means that MD can be used to simulate any compositions, even involving chemicals that have never been synthesized.
While MD is a well-established technique long used in academic research as well as in the pharmaceuticals industry, it has not yet fully established itself in most industrial use-cases, including battery modelling. This is because industrial requirements on simulations differ markedly from academic ones. To be useful for industrial applications, simulation workflows need to be accurate and computationally cheap at the same time, and the process should be labor efficient and reproducible.
Enter Compular Lab
At Compular, our answer to all these requirements is Compular Lab, a web application that lets users very simply specify the electrolytes they would like to simulate and automates everything else. Compular Lab relies on the highest-quality simulation techniques for maximum accuracy, and runs them in the cloud with optimal hardware and simulation settings to make it affordable and scalable. For a validation of our simulation quality, see our recently published whitepaper.
In this case, Compular’s simulation capabilities have been used to capture the change in electrolyte properties as a function of local salt concentration and EC solvent fraction, but this also generalizes to e.g. temperature effects or changing electrolyte components altogether. If you have a well-parameterized cell model for any given cell design, the combination of Compular Lab and COMSOL Multiphysics can enable you to simulate how the properties of this cell would change if you made a drop-in replacement of the electrolyte, making this a very efficient tool for early-stage cell development. And unlike labs and pilot lines, cloud computing is extremely scalable. A hundred simulations can be completed in the same time as one, and without lead times or investments beyond the cost of running the simulations.
What did we learn?
Compular Lab was used to predict ionic conductivity, salt diffusivity, thermodynamic factor, and Li+ transference number.
Figure 1 shows our predictions for salt conductivity. We observed nonlinear dependence on both salt concentration and EC fraction of solvent. The conductivity increases with salt concentration as long as it is low, due to the increase in the number of charge carriers. As the salt concentration increases beyond a Goldilocks zone, which is different for each EC fraction, the conductivity starts to decrease with salt concentration, due to a higher degree of ion pairing and aggregation, as well as an overall increase of viscosity.
Conductivity decreases as EC is consumed. This is somewhat counterintuitive, since as we can see in Figure 2, the salt diffusivity monotonically increases as EC is consumed. The explanation for this behavior is that EC is a stronger solvent of LiPF6 than EMC, which means that while each ion becomes more mobile as the fraction of EC goes down, it also becomes more prone to coordinating with its counter-ion so that they form neutral ion pairs and the net charge carrier concentration goes down. We do, however, see a reversal at the highest salt concentrations, where lower EC content eventually leads to higher conductivity due to the high viscosity at high salt concentrations.

Figure 1. Ionic conductivity as a function of salt concentrations for different EC percentages of the solvent, as computed using Compular Lab. There are nonlinear effects of both salt concentration and EC fraction, which are represented by a semiempirical surface fit.
Salt diffusivity decreases nonlinearly with salt concentration, with a high initial drop followed by a slower decrease as the concentration increases (Figure 2). This reflects the transition from the dilute regime, where ions interact weakly, to the concentrated regime, where ion-ion interactions are an important impediment to diffusivity. As EC is consumed, diffusivity increases linearly.

Figure 2. Salt diffusivity as a function of salt concentration for different EC percentages of the solvent, as computed using Compular Lab. There is a nonlinear effect of salt concentration and a linear dependence on EC fraction, which are represented by a semiempirical surface fit.
The thermodynamic factor (Figure 3), which is related to the change of activity coefficient as concentration increases, and can be understood as a measure of non-ideality of the electrolyte when it deviates from 1, increases linearly with salt concentration, and has no discernible dependence on EC content.

Figure 3. Thermodynamic factor as a function of salt concentration for different EC percentages of total solvent as computed from Compular Lab. The line represents a linear fit used for all EC fractions.
Finally, the cation transference number was found to be 0.31 irrespective of both salt concentration and EC fraction.
What difference does this make on the cell level?
Our partners at COMSOL set up and ran P2D simulations with aging with and without the effects of solvent composition on electrolyte rheology, in order to study the effects on overall cell behavior.
The most direct impact on the cell behavior was that including the increase of salt diffusivity with aging strongly suppressed the salt concentration gradient across the cell, which was especially pronounced at the end of a discharge (Figure 4). This clearly illustrates that including these effects is crucial for capturing the correct behavior of battery cells as they age.

Figure 4. Salt concentration gradients at the end of discharge with and without accounting for the effect of EC loss on electrolyte transport, as computed using COMSOL Multiphysics.
The upshot of this difference in concentration gradient is a lower cell polarization throughout the second half of the cycle, compared to what would be predicted without the effect of changing solvent composition, at a maximum 40 mV difference at 90% SOH (Figure 5).

Figure 5. Cell polarization as a function of depth of discharge for the pristine cell (100% SOH), and at 90% state-of-health (SOH) with and without accounting for changing electrolyte properties due to EC consumption, as computed by COMSOL Multiphysics. Towards the end of the discharge, there is a meaningful difference in cell polarization.
While this may not seem like a very big difference, it has a very meaningful impact on the number of cycles until reaching 90% SOH (Figure 6). This takes 1000 cycles when the transport effects of the solvent composition change is taken into account, compared to 800 when it is not, or a 25% increase in predicted number of cycles until 90% SOH. As can be seen in Figure 6, this gap becomes bigger the longer the cell is cycled, such that the difference will be even more pronounced at the end-of-life (EOL), typically defined as 80% SOH or less.

Figure 6. Discharge capacity at 1C relative to the pristine cell with and without accounting for the changes of electrolyte properties due to loss of EC, as computed by COMSOL Multiphysics. When these effects are included, the cell takes 200 more cycles to reach 90% SOH. This is a 25% increase in cycle life compared to when not accounting for EC consumption.
Summary
We combined MD simulations in Compular Lab with P2D modeling with aging in COMSOL Multiphysics to explore the impact of changing electrolyte transport properties due to EC breakdown on cell behavior and performance. We found that including these effects is necessary to get a correct description of the cell, and that they have a meaningful impact not only on cell polarization and salt gradients, but also on the cycle life of the cell.
While including such effects in cell models is highly advisable for these reasons, determining the electrolyte properties as functions of salt concentration and solvent composition is challenging, unless they can be predicted from first principles. Compular Lab allows this to be done simply and scalably with minimal onboarding required from the user.
Want to learn more?
If you would like to try out Compular Lab for parameterizing your cell models, or for screening formulations, contact us at .
If you would like to read more about how the cell model was set up in COMSOL Multiphysics, or play around with the model yourself, read COMSOL’s blog post about this project here.